WHAT WE DO
At IEM Health, our team of medical, public health, and program management experts have an in-depth understanding of the different COVID-19 vaccines available, those in the pipeline and the journey a vaccine goes through from development to distribution. IEM Health’s “COVID-19 Vaccine Working Group” started analyzing and developing vaccine management solutions for in the early fall of 2020.
The vaccine journey from development to manufacturing and distribution is usually a prolonged process that takes years to perfect, test, and distribute. The development of a potential Coronavirus vaccine under normal circumstances would take around ten years to perform the research, undergo multiple clinical trials, and receive government approval. However, due to the deadliness and severity of COVID-19, a vaccine for the virus required an unprecedented quickening of the process to save lives.
Under the Trump Administration, Operation Warp Speed (OWS) was responsible for the acceleration of testing, development, and distribution of diagnostics, therapeutics, and vaccines, to combat COVID-19. OWS’s mission was to ensure that a vaccine will be produced as rapidly as possible while adhering to standards of safety and efficacy. While OWS accelerated the process, it did not eliminate steps. Rather the program allowed steps to occur simultaneously, such as starting mass manufacturing of a vaccine before a vaccine is finished with clinical trials, permitting the vaccine to progress faster without compromising the safety of the product. Protocols established by the federal government, rather than public-private partnerships also help ensure the vaccine is produced safely and rapidly.
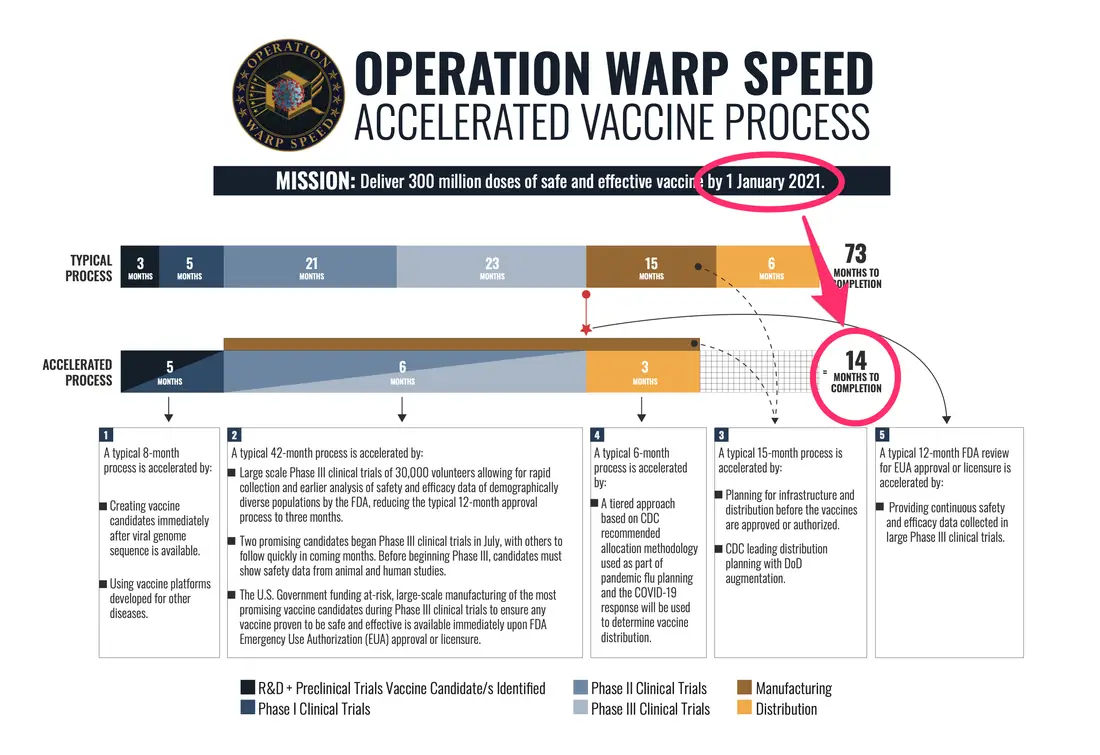
(Source: www.defense.gov/Explore/Spotlight/Coronavirus/Operation-Warp-Speed/)
On January 21, 2021 the Biden Administration launched a new comprehensive strategy to combat the COVID-19 pandemic with the goal of vaccinating 100 million Americans in 100 days. The mission of OWS was to deliver over 300 million doses of a safe and effective vaccine to 150 million Americans by January 2021 as noted in Figure 1 above. Although there have been some delays and challenges, the national strategies have been highly successful – leading to the creation and distribution of multiple COVID-19 vaccines and countermeasures at record speed. This is astonishing given the fact that no vaccine or therapeutic has been developed during an exploding pandemic.
The five leading vaccines approved or being evaluated in the United States are produced by Pfizer/BioNTech, Moderna, AstraZeneca/Oxford, J&J (Jassen), and Novavax. The timeline for the vacancies is shown in Figure 2 below.
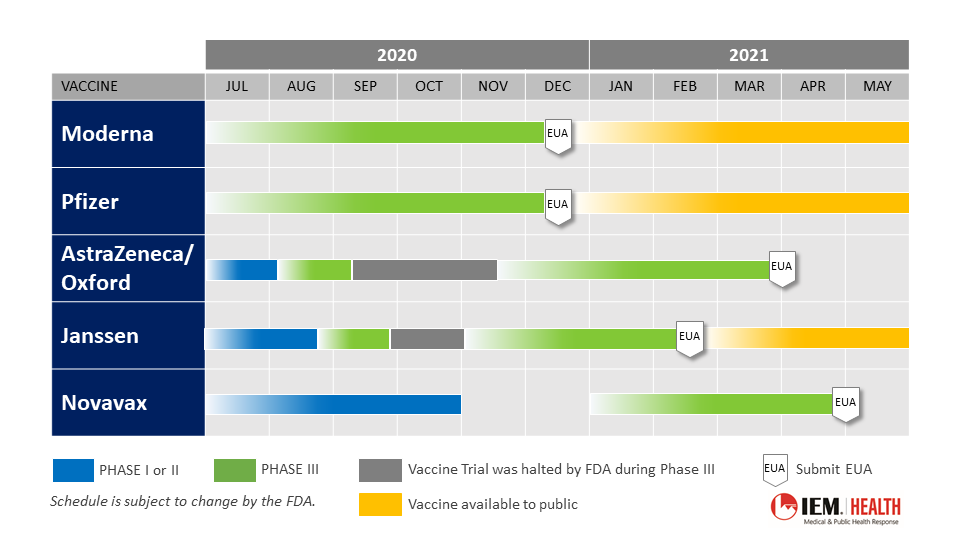
- Pfizer/BioNTech: Pfizer/BioNTech’s Phase 3 clinical trial for its mRNA vaccine included over 43,000 volunteers. The trial found the coronavirus vaccine was 95% effective in preventing infections and submitted its results to the U.S. Food & Drug Administration (FDA) for emergency use on November 20, 2020. Following the confirmation of the safety and efficacy of the vaccine, the FDA advisory panel voted on December 10, 2020 to endorse the vaccine and granted it an Emergency Use Authorization (EUA) on December 11, 2020 for people 16 years and older. With an FDA EUA granted, the first vaccinations in the United States. were administered on December 14, 2020. In the United Kingdom, the Medicines and Healthcare products Regulatory Agency (MHRA), the equivalent of the U.S. FDA gave the green light to the vaccine on December 2, 2020 and vaccinations began on December 8, 2020. This vaccine requires two doses and must be stored at a temperature of minus 700 However, it can be stored at 20 to 80 centigrade for up to 5 days. Pfizer/BioNTech has promised the delivery of 60 million shots to America by the end of March 2021[1]. The companies also announced a study to develop a booster shot for the B.1.351 variant.
- Moderna: Moderna submitted its vaccine to the FDA for an EUA on November 30, 2020 to review data showing the vaccine is 94.5% effective at preventing Covid-19 and 100% effective at preventing severe cases of the disease. The FDA granted the vaccine an EUA on December 18, 2020 for people 18 years and older, prompting vaccinations to begin on December 21, 2020. This vaccine also requires two doses and must be stored at a temperature of minus 200 However, it can be stored at 20 to 80 centigrade for up to 30 days. Moderna has promised the delivery of 50 million shots to America by the end of March 2021. The company also began two new trials for a vaccine against the B.1.351 variant and a new, refrigerator-stable COVID-19 vaccine, both vaccines are in Phase 1 trials.
- AstraZeneca/Oxford: AstraZeneca’s Phase 3 of its viral vector (non-replicating) Covid-19 vaccine trial with 23,000 volunteers found that the vaccine was 70% effective in its first half of the dose and up to 82.4% effective after the second dose. The vaccine is proven to be less expensive and involve fewer steps in distribution and administration compared to Moderna and Pfizer’s vaccines. However, there were some errors in data reporting that might result in a few months delays for an FDA EUA submission, with their submission for FDA approval expected to come in late-March to early-April 2021.
- Johnson & Johnson: Janssen (Johnson & Johnson) submitted its vaccine to the FDA on February 4, 2021 and received an EUA on February 27, 2021. On February 2 the FDA released an analysis of the Janssesn trials concluding the vaccine had an 70% efficacy. This is a single dose vaccine and must be stored at minus 20O. However, it can be safely stored at 20 to 80 centigrade for 3 months. In the initial rollout of the vaccine Johnson & Johnson provided 4 million doses to the United States and the company plans to deliver a total of 20 million doses by April 2021.
- Novavax: Novavax completed enrollment in Phase 3 trials of its protein subunit vaccine in the U.S. and Mexico in mid-February and is expected to deliver results in April 2021. The biotechnology company is hoping to submit its vaccine to the FDA for EUA in May. This is a two-dose vaccine and can be stored at 20 to 80 centigrade. Novavax reported their phase 3 trials in the United Kingdom showed 96% efficacy against the original coronavirus and 55% efficacy against the South Africa variant. If the clinical trial is successful and Novavax is granted EUA, the company expects to deliver 100 million doses for use in the U.S. in 2021.
Steps in the Vaccine Journey include…
- Discovery and pre-clinical – In the first stage of a trial, researchers analyze the organism and decide the optimal method for developing a vaccine. This includes understanding the structure of the organism, how it enters the body, and how the immune system responds at a molecular level. Once an initial vaccine is developed it is then tested on animals in a laboratory.
- Clinical Trials – If a vaccine shows favorable results in the pre-clinical stage it can then enter clinical trials and be tested on humans. The COVID-19 studies are randomized, double-blinded, placebo-controlled clinical trials, meaning some participants receive the COVID-19 vaccine while others will get a placebo. There are three phases of clinical trials a vaccine must pass:
- Phase 1 Trials – The vaccine is tested on a small group of healthy adults (less than 100) who are monitored carefully for any adverse symptoms or immune response. This phase also seeks to determine the most effective dosage.
- Phase 2 Trials – The vaccine is tested on a larger group of people (several hundred) and the immune response is observed more closely.
- Phase 3 Trials – Thousands of individuals receive the vaccine or a placebo, and the immune response is observed to determine vaccine safety, most common side effects, and efficacy.
- Developer applies for an Emergency Use Authorization – The FDA convenes an advisory committee of infectious disease experts, the Vaccines and Related Biological Products Advisory Committee (VRBPAC), to review the trial data and submits an official recommendation to the FDA. If the FDA approves the vaccine or grants an emergency use authorization, the Centers for Disease Control and Prevention (CDC) will then convene a separate committee, the Advisory Committee on Immunization Practices (ACIP), to review the data and submit recommendations on when and how the vaccine should be administered to different populations.
- Manufacturing and procurement – Developers must decide how many vaccine doses will be needed for widespread distribution, and plan for how many doses will be necessary in each phase of distribution. Manufacturing a nation-wide vaccine involves finding a proper facility and acquiring all the necessary resources such as proper storage containers, glass vials, and safety equipment.
- Distribution – Operation Warp Speed developed a comprehensive plan for a centralized phased distribution of the COVID-19 vaccines that will be carried out by the federal government, all 50 states, localities, U.S. territories, tribes, industry partners, and other entities. Successful implementation of the national COVID-19 vaccination program requires precise coordination among all of these many public and private partners. Cooperation on each of these fronts has already begun, as detailed in HHS’s strategy document.
When vaccines first became available only a limited number of doses were available but as of March 21, 2021, 127 million doses have been administered in the United States and 13.5% of the population is fully vaccinated. The Nation. currently leads the world in total vaccines administered and is averaging 2.44 million doses administered every day. Vaccine manufacturers have promised enough shots to fully vaccinate 130 million Americans by the end of March 2021. Assuming drug makers are successful in meeting their delivery targets, 400 million Americans will be fully vaccinated by the end of July 2021. These numbers could increase if drug makers are able to safely increase production at their manufacturing facilities or if another vaccine is approved in the coming months.
The IEM Modeling and Emerging Technology teams developed a visualization dashboard so you can see what the COVID-19 pandemic might look like in the absence of any vaccinations and compare that against scenarios where we can meet the goals of administering 100 million or 150 million doses of vaccine in 100 days. Users can see how many confirmed COVID-19 cases and deaths could be averted under these circumstances as well as potential consequences if the vaccine administration rate is slower or faster than planned. Given the many uncertainties surrounding the COVID-19 pandemic, our projections for new COVID-19 cases and deaths are not meant to imply quantitative accuracy, rather they provide a way to see the relative benefits of different vaccination administration rates or different vaccination strategies.
Nationwide Distribution and Prioritization
The COVID-19 vaccination program requires a phased approach due to limited supply. Throughout distribution, recipients are monitored for adverse symptoms and immune response. The CDC recommends that public health officials identify the critical and at-risk populations in their communities to best determine who should receive the vaccine first.
On Tuesday, December 1, 2020, the ACIP recommended health care personnel and residents of nursing homes and similar facilities be the first to receive the vaccine in “Phase 1a” of the nationwide distribution.
Vaccine advisers to the CDC voted 13-1 on December 1, 2020, to recommend that both healthcare workers and residents of long-term care facilities be first in line for any coronavirus vaccines that get emergency authorization from the FDA. The ACIP voted to include both groups in what they’re calling Phase 1a of the CDC’s coronavirus vaccine distribution plan.
After vaccinating healthcare personnel and residents of nursing homes, states have widened the pool of eligible recipients to include essential workers, teachers, all individuals over the age of 65, as well as high risk individuals 64 and younger. All states are vaccinating health care workers and residents of nursing homes and most states have expanded eligibility to teachers and high-risk adults.
On March 13, 2021, President Biden announced that the Administration will direct states to make every adult in the U.S. eligible for vaccination by May 1.
The federal government has delivered approximately 142.9 million doses to states, territories and federal agencies. While states receive their vaccine supply from the federal government, each state and territory is responsible for creating and implementing plans for vaccine prioritization and distribution. Some states have been more efficient than others in administering the vaccine due to varying demand, lags in data reporting and other logistical challenges.
The Biden Administration’s National Strategy for the COVID-19 Response and Pandemic Preparedness
Throughout the distribution process increasing access to the vaccine is essential and the Biden Administration is prioritizing equitable access in the new comprehensive strategy.
The Biden Administration has directed states, Tribes, and territories to make every adult in the United States eligible for the vaccine by May 1. To facilitate the vaccination of every adult American the administration plans to:
- Deliver vaccines directly to up to an additional 700 community health centers that reach underserved communities;
- Double the number of pharmacies participating in the federal pharmacy program;
- More than double the number of federally-run mass vaccination centers, run by FEMA, the U.S. military, and other federal agencies in partnership with states; and
- Deploy more than 4,000 active duty troops to support vaccination efforts.
The CDC also launched VaccineFinder to help Americans find the most up-to-date information on COVID-19 vaccine availability and help everyone who wants a vaccine get vaccinated.
The information contained in IEM’s “A Vaccine’s Journey” is current as of 3:00 p.m. ET on March 22, 2021.
[1] https://www.bloomberg.com/news/articles/2021-02-23/more-vaccine-doses-they-re-coming-say-u-s-drugmakers?sref=Pvx4pkAx
[1] Pfizer and BioNTech to Submit Emergency Use Authorization Request Today to the U.S. FDA for COVID-19 Vaccine, November 20, 2020. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-submit-emergency-use-authorization.
[2] Moderna Announces Primary Efficacy Analysis in Phase 3 COVE Study for Its COVID-19 Vaccine Candidate and Filing Today with U.S. FDA for Emergency Use Authorization. November 30, 2020. https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-primary-efficacy-analysis-phase-3-cove-study.
[3] National Academies of Sciences, Engineering, and Medicine. Framework for Equitable Allocation of COVID-19 Vaccine. Washington, DC: The National Academies Press, 2020. https://doi.org/10.17226/25917.




